First you need to understand how medical device development from prototype to regulatory approval works.
Medical device development from prototype to regulatory approval.
Confidential not for circulation.
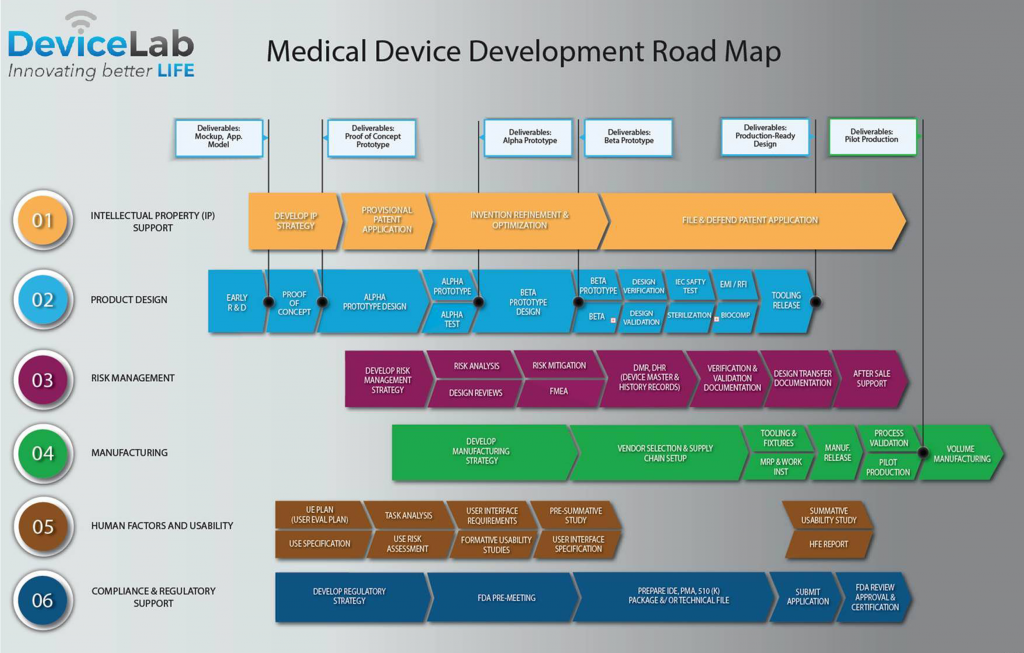
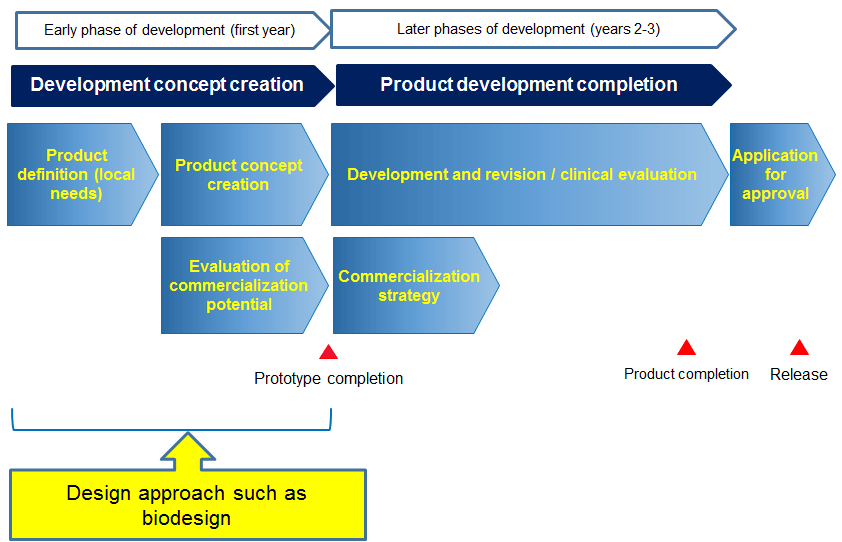
The development path follows a certain route from device conception intellectual property generation and testing to regulatory approval.
In the early part of the 20th century the u s.
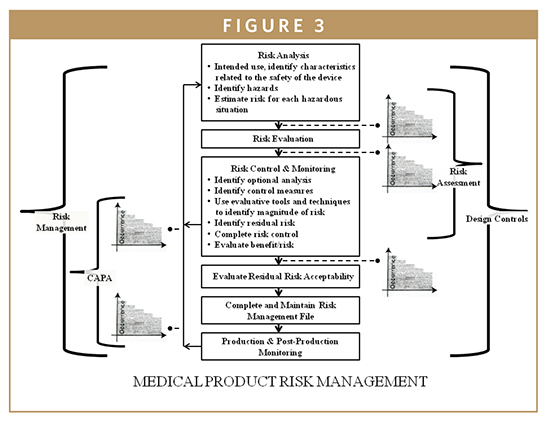
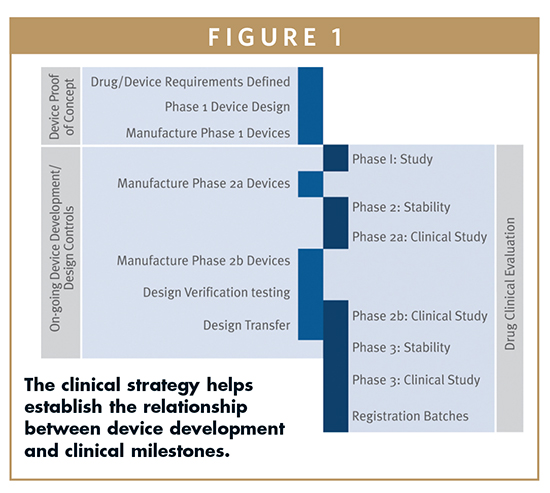
Our maturity in medical device development design cycle brings in traceability in all process there by easier regulatory approvals and faster time to market.
Food and drug administration fda was given the responsibility for ensuring both the safety and efficacy of drugs prior to marketing amendments to the federal food drug and cosmetics act in 1976 expanded the agency s role to oversee safety in the development of medical devices whereas new drug approval takes an average of 12 years moving new.
Medical device development from prototype to regulatory approval.
Here are the phases you must pass through to have your medical device breakthrough hit the open market.
Since cardiac medical devices are created to help.
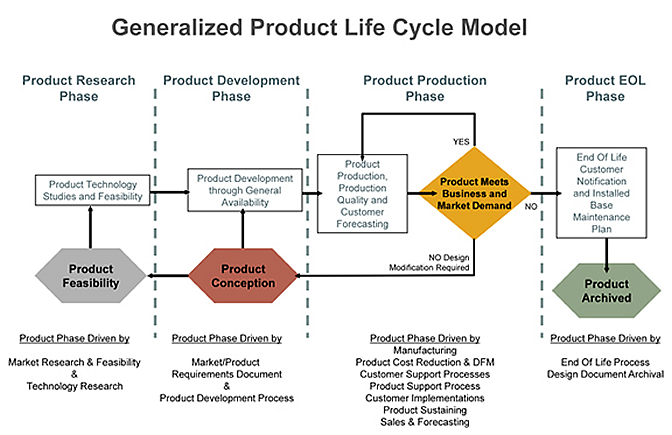
Medical device product launch.
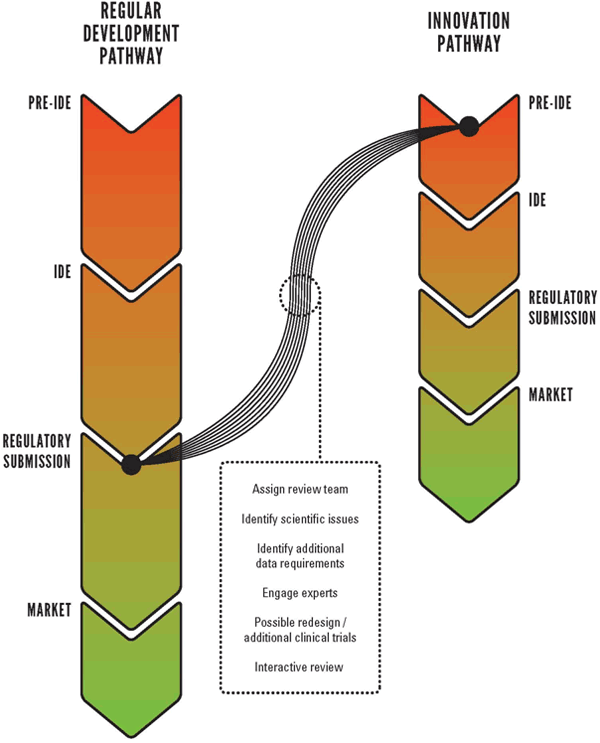
Regulatory7 path outlined clear regulatory path fda approval major company acquisition quality work risks milestone based planning risk 1 10 risk 1 10 value revenues risk 1 10.
The path to commercialization of a medical device is long expensive and takes an engineering mindset.
Thus our medical device engineering services team can take care the complete cycle of medical device development from prototype to regulatory approval.
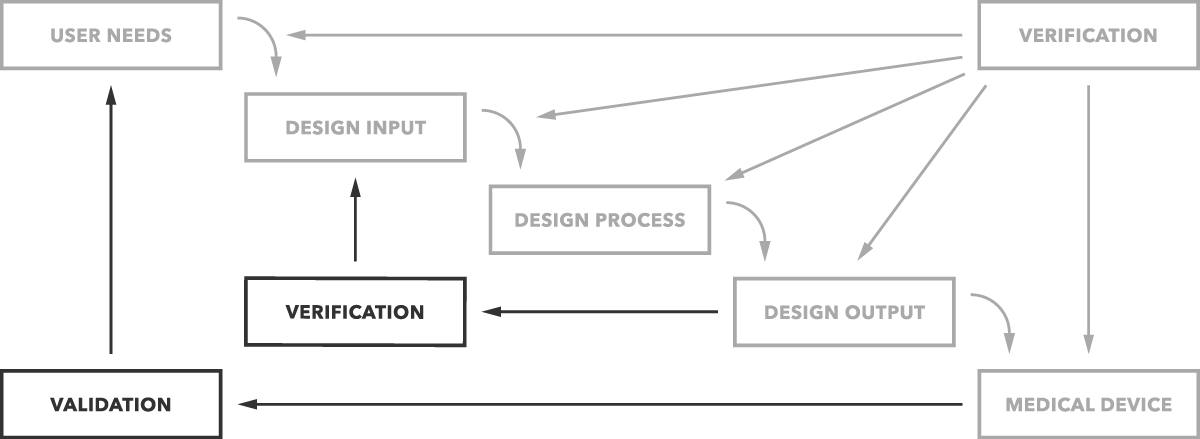
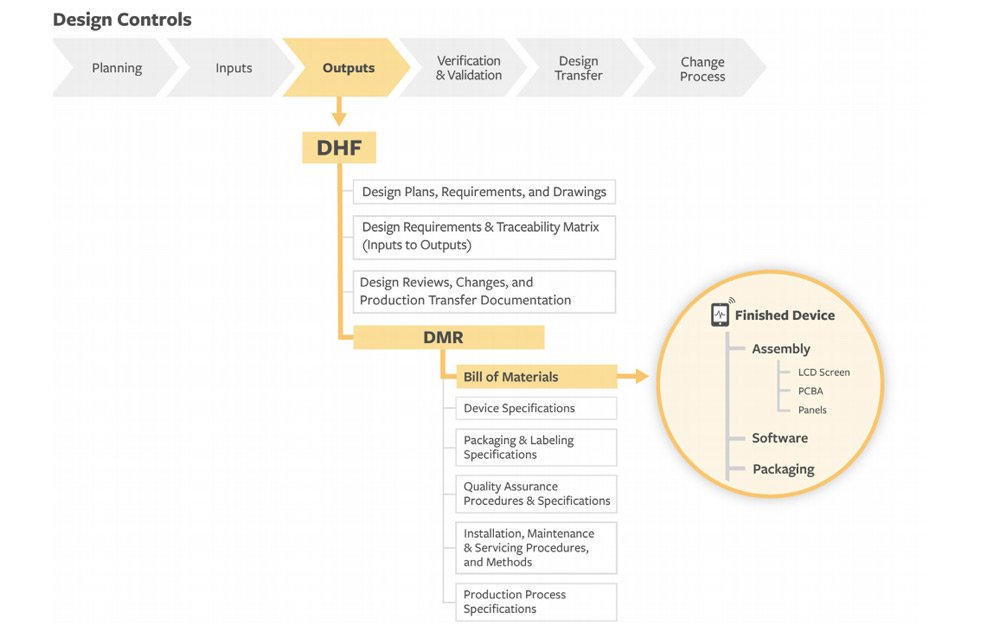
Strict design controls for the medical device industry are in place meaning an expert team is not enough to get regulatory approval for a medical device.
Our experts train hospital and critical care staff in device.
At least according to the authorities assessing market approval.